
Introducing Blue Pen RX, your GLP-1 source.
Get your patients the quality medications they need at a price that will make you both very happy.
Our Mission
Our mission, in collaboration with BPI Labs, is to enhance the affordability and accessibility of life-changing medications. We are committed to ensuring these vital medications reach the physicians, pharmacies, and patients who need them most.
BPI Labs has leveraged its manufacturing scale to give patients access to the medications they deserve.
The Blue Pen RX Difference
We Save you Time and Money!
Because our products do not require reconstitution, it saves both time and effort for you and your patients. This also minimizes the potential for dosing errors.
Licensed 503B Facility!
Our manufacturing facility adheres to FDA cGMP standards and operates under a 503B license, ensuring the highest level of quality and safety. Our Semaglutide is assigned an FDA National Drug Code (NDC) Number, facilitating easy identification and tracking by prescribers to ensure patients receive the correct treatment.
Because Quality Matters!
Our products are pure GLP-1s. We do not combine it with untested ingredients such as peptides or B12, as there is no scientific evidence supporting the safety and efficacy of such mixtures.
Fresh Not Freeze Dried!
Our products are delivered reconstituted and not lyophilized, meaning it is not freeze-dried. We believe that avoiding the freeze-drying process helps maintain the quality and efficacy of GLP-1s. Additionally, our streamlined packaging reduces material use and lowers our carbon footprint.
Because Consistency Matters!
Our FDA-registered manufacturing facility, also a 503B facility, is capable of producing large, consistent quantities, offering significant cost savings to our healthcare partners and customers.
Safety is THE Priority!
Our team consists of top professionals in R&D, Medical Affairs, Pharmacy, Quality, Engineering, and more. We are prepared to collaborate with you to develop safe and effective medications tailored for office-use dispensing.
Why Blue Pen RX?
-
We Are Here to Help Meet Your Needs
-

Base Form Ingredients
-
Fast Afforable Shipping
-
503b Licensed Facility
Our Semaglutide
-
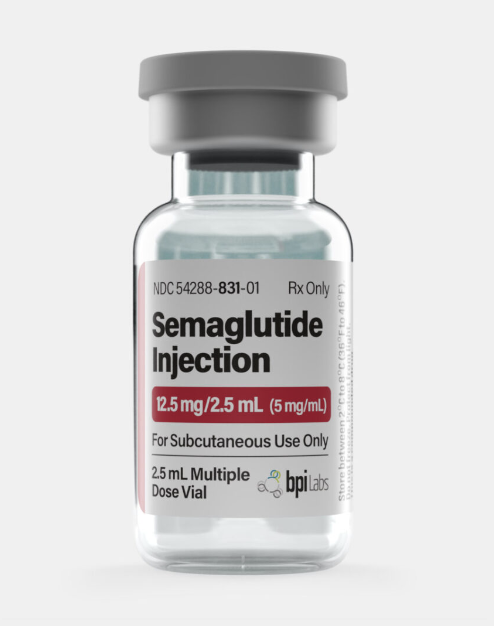
12.5 mg/2.5 mL
-

5 mg/2 mL
-
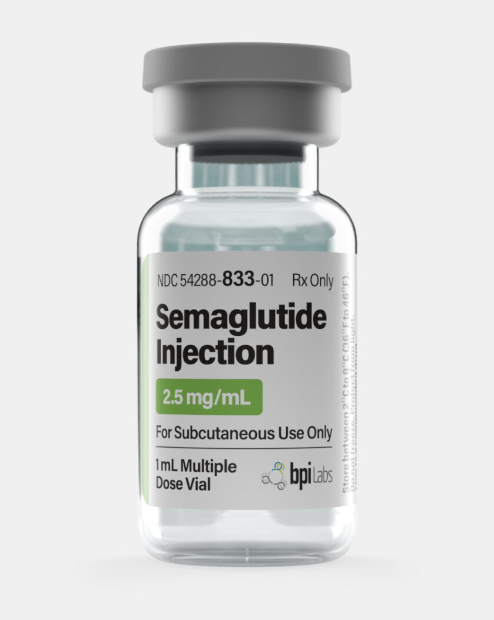
2.5 mg/mL
-

1 mg/mL
“Blue Pen RX is here to help medical professionals help their patients. We take great pride to ensure patients and doctors get access to the medicine they need to improve their quality of life!”
— BERT R., BLUE PEN RX - VP OF SALES

